What’s rust? – Henry E., age 13, Boston, Massachusetts
Think about leaving your shiny metallic bicycle exterior within the rain. As water swimming pools on its surfaces, oxygen from the air lingers close by, and collectively they start to quietly assault the metallic.
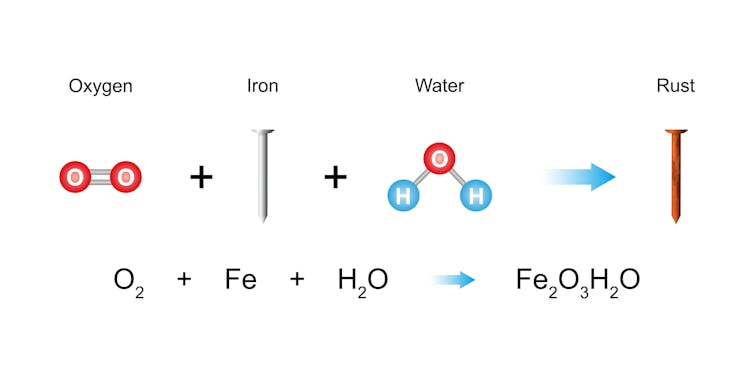
The iron within the bike and the oxygen and water within the atmosphere collectively endure a chemical response. It types iron oxide – higher often known as rust – which accumulates over time. This reddish-brown, flaky substance is extra than simply ugly; it’s an indication that the metallic is breaking down.
Iron reacts with oxygen and water to kind rust, an oxidized type of iron.
Ali Damouh/Science Photograph Library by way of Getty Photos
Chemists name this course of oxidation. You’ll be able to consider iron as like a superhero — robust, sturdy and glossy — till it meets its kryptonite: moisture and air. Water helps iron atoms extra simply shed their electrons, that are negatively charged particles. Oxygen acts like a tiny electron thief, stealing these electrons and leaving the metallic weak and crumbly.
The shiny, metallic iron utilized in houses and business is a refined kind of what’s present in nature — iron ore. Rust is a pure course of — the refined iron is basically making an attempt to return to its unique oxidized, secure state: iron ore.

Outdated water pipes can clog with amassed rust.
chimmy/E+ by way of Getty Photos
From bikes and vehicles to bridges and ships
From family fixtures to monumental machines, rust strikes in wherever metallic meets moisture and time.
On bikes and vehicles, the mixture of rain and uncovered metallic usually triggers a full-blown rust social gathering, consuming away at frames and undercarriages. Outdated water pipes are one other scorching spot — over time, they corrode from the within, usually leaking brown, rusty water into sinks and tubs. Within the kitchen, standing water left round sinks or taps can result in yellow-orange rust stains which are as cussed to take away as they’re ugly.
On a a lot bigger scale, rust can wreak havoc on ships and bridges. Corroded hulls can result in oil leaks and even catastrophic sinking, costing the maritime business billions of {dollars} every year in repairs and environmental injury.
And right here’s a twist – salt hurries up the rusting course of. In snowy areas, highway salt doesn’t simply soften ice; it additionally turbocharges oxidation, accelerating the corrosion course of. That’s why vehicles in snowy locations would possibly rust sooner.

The copper Statue of Liberty is greenish resulting from oxidization, which types the colourful patina, the copper model of rust.
ErickN/iStock by way of Getty Photos Plus
Rust is the time period for the particular sort of corrosion that happens in iron or metal. However any metallic can chemically degrade resulting from reactions with environmental elements like oxygen and moisture.
Even outdated statues aren’t immune. These greenish-blue figures you see in parks and plazas? That’s not paint; it’s patina, the copper model of rust. Although extra visually interesting, patina continues to be a type of corrosion.
How will you cease rust?
By understanding the chemistry behind rust, folks have discovered sensible methods to sluggish it down and even cease it altogether, defending all the pieces from bridges to bicycles.
One of many easiest strategies is to color the metallic floor. Paint acts like a water-resistant jacket, sealing the metallic off from air and moisture – the 2 key components for rust.
Conserving metallic dry is one other sensible protection. Instruments are sometimes saved in dry areas or alongside dehumidifiers to attenuate moisture.
One other method, known as galvanization, coats one metallic with a extra reactive one, like zinc. Zinc corrodes extra simply than iron, forming a secure layer of zinc oxide when uncovered to air and moisture. The zinc oxide is a protecting barrier to additional corrosion – till it regularly wears away, leaving the underlying iron susceptible to rust as soon as once more.
After which there’s chrome steel: a corrosion-resistant combination, known as an alloy, of iron with different metals, comparable to chromium. When uncovered to air, chromium undergoes its personal model of rusting, forming a secure layer of chromium oxide.
This layer is extraordinarily skinny, 100,000 instances thinner than a single strand of human hair. It’s invisible to the bare eye, sticks tightly to the metallic floor and prevents additional oxidation. The chromium oxide layer can be self-healing — if scratched, the uncovered chromium rapidly reforms the protecting barrier. That’s why chrome steel is utilized in all the pieces from kitchen sinks to surgical instruments.
Watching rust on the atomic stage
I’m a supplies scientist who makes use of superior imaging instruments, like transmission electron microscopes, to review how metals oxidize on the atomic scale. We will really watch as tiny metallic atoms lose electrons and oxygen atoms acquire them, present process corrosion in actual time.
Transmission electron microscopy video reveals the real-time floor oxidation of aluminum. To start with, the small dots are particular person aluminum atoms, neatly lined up in an everyday sample. Oxygen from the water vapor sticks to the floor and mixes with the aluminum. A brand new layer of aluminum oxide types, with the atoms organized in a messy random approach. This new floor layer protects the aluminum beneath, which stays the identical.
As an illustration, in a single examine, we noticed the best way a skinny layer of aluminum oxide types immediately when aluminum is uncovered to oxygen or water vapor. This oxide layer doesn’t flake off like rust on iron; as a substitute, it types a uniform, tightly bonded protecting coating that blocks any extra oxygen and water from reaching the underlying metallic. That’s why aluminum resists continued corrosion – not like iron, whose rust layer is porous, permitting the response to maintain going.
Oxidation might be dangerous, inflicting rusting, or helpful when harnessed to guard metals as a substitute. By observing these processes in actual time, my colleagues and I goal to create supplies that last more and work higher in on a regular basis life — for instance, to assemble stronger bridges, extra environment friendly batteries and safer airplanes.
And since curiosity has no age restrict – adults, tell us what you’re questioning, too. We received’t be capable to reply each query, however we are going to do our greatest.



