How does cleaning soap clear our our bodies? – Charlie H., age 8, Stamford, Connecticut
Hundreds of years in the past, our ancestors found one thing that may clear their our bodies and garments. Because the story goes, fats from somebody’s meal fell into the leftover ashes of a fireplace. They had been astonished to find that the mixing of fats and ashes shaped a cloth that cleaned issues. On the time, it will need to have appeared like magic.
That’s the legend, anyway. Nevertheless it occurred, the invention of cleaning soap dates again roughly 5,000 years, to the traditional metropolis of Babylon in what was southern Mesopotamia – right this moment, the nation of Iraq.
Because the centuries handed, folks all over the world started to make use of cleaning soap to wash the issues that obtained soiled. Throughout the 1600s, cleaning soap was a typical merchandise within the American colonies, usually made at house. In 1791, Nicholas Leblanc, a French chemist, patented the primary soapmaking course of. Right now, the world spends about US$50 billion yearly on tub, kitchen and laundry cleaning soap.
However though billions of individuals use cleaning soap each day, most of us don’t know the way it works. As a professor of chemistry, I can clarify the science of cleaning soap – and why you must take heed to your mother when she tells you to scrub up.
You’ll be amazed at how a lot work it takes to make a bar of cleaning soap.
The chemistry of fresh
Water – scientific title: dihydrogen monoxide – consists of two hydrogen atoms and one oxygen atom. That molecule is required for all life on our planet.
Chemists categorize different molecules which are interested in water as hydrophilic, which suggests water-loving. Hydrophilic molecules can dissolve in water.
So in case you had been to scrub your palms below a working faucet with out utilizing cleaning soap, you’d in all probability eliminate a lot of no matter hydrophilic bits are caught to your pores and skin.
However there’s one other class of molecules that chemists name hydrophobic, which suggests water-fearing. Hydrophobic molecules don’t dissolve in water.
Oil is an instance of one thing that’s hydrophobic. You in all probability know from expertise that oil and water simply don’t combine. Image shaking up a jar of French dressing salad dressing – the oil and the opposite watery substances by no means keep combined.
So simply swishing your palms by way of water isn’t going to eliminate water-fearing molecules akin to oil or grease.
Right here’s the place cleaning soap is available in to save lots of the day.
Cleaning soap, a fancy molecule, is each water-loving and water-fearing. Formed like a tadpole, the cleaning soap molecule has a spherical head and lengthy tail; the top is hydrophilic, and the tail is hydrophobic. This high quality is among the causes cleaning soap is slippery.
It’s additionally what provides cleaning soap its cleansing superpower.
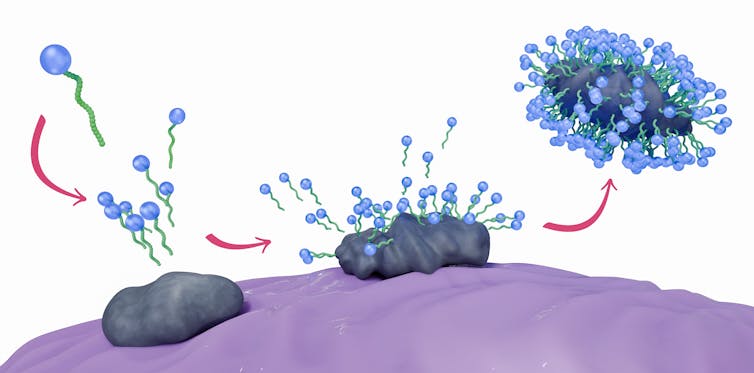
The spherical head and lengthy wiggly tail of the cleaning soap molecules work collectively to eradicate dust, grease and dirt.
Tumeggy/Science Picture Library by way of Getty Photos
A microscopic view
To see what occurs while you wash your palms with cleaning soap and water, let’s zoom in.
Image all of the gunk that you simply contact through the day and that builds up in your pores and skin to make your palms soiled. Possibly there are smears of meals, mud from exterior, and even sweat and oils from your individual pores and skin.
All of that materials is both water-loving or water-fearing on the molecular stage. Grime is a jumbled mess of each. Mud and useless pores and skin cells are hydrophilic; naturally occurring oils are hydrophobic; and environmental particles might be both.
When you use solely water to wash your palms, lots shall be left behind since you’d solely take away the water-loving bits that dissolve in water.
However while you add a little bit of cleaning soap, it’s a distinct story, due to its concurrently water-loving and water-fearing properties.
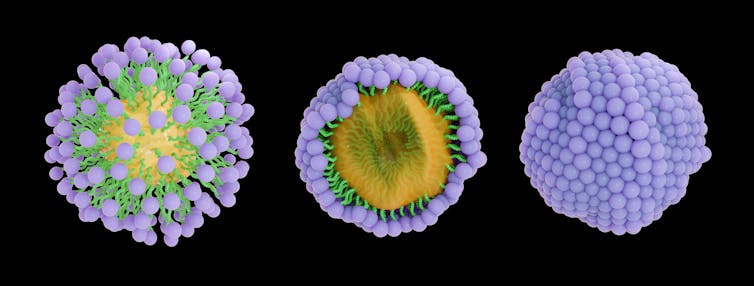
Cleaning soap molecules work collectively to encapsulate grime inside a bubblelike micelle construction.
TUMEGGY/Science Picture Library by way of Getty Photos
Cleaning soap molecules come collectively and encompass the grime in your palms, forming what’s often known as a micelle construction. On a molecular stage, it seems nearly like a bubble encasing the hydrophobic little bit of particles. The water-loving heads of the cleaning soap molecules are on the floor, with the water-fearing tails contained in the micelle. This construction traps the dust, and working water washes all of it away.
To get the complete impact, wash your palms on the sink for no less than 20 seconds. Rubbing your palms collectively helps power the cleaning soap molecules into no matter dust is there to interrupt it up and envelope it.
It’s not simply dust
Together with dust, your physique is roofed by microorganisms – micro organism, viruses and fungi. Most are innocent and a few even shield you from getting sick. However some microorganisms, often known as pathogens, may cause sickness and illness.

Whether or not liquid or bar, cleaning soap will get the job achieved.
velvelvel/iStock by way of Getty Photos Plus
They’ll additionally trigger you to odor in case you haven’t taken a shower shortly. These micro organism break down natural molecules and launch smelly fumes.
Though microorganisms are protected by a barrier – it’s referred to as a membrane – cleaning soap and water can disrupt the membrane, inflicting the microorganism to burst open. The water then washes the stays of the microorganism away, together with the stink.
To say that cleaning soap modified the course of civilization is an understatement. For hundreds of years, it’s helped preserve billions of individuals wholesome. Consider that the subsequent time Mother or Dad asks you to scrub up – which is able to seemingly be someday quickly.
And since curiosity has no age restrict – adults, tell us what you’re questioning, too. We gained’t have the ability to reply each query, however we’ll do our greatest.



